Patient safety in medical research has become a critical priority amid ongoing challenges, particularly with recent funding cuts affecting vital oversight systems. As research grant freezes disrupt the work of institutional review boards (IRBs), the protection of participants’ rights and welfare is jeopardized. The significance of IRB patient protection cannot be overstated, as they ensure compliance with ethical standards and legal regulations throughout research studies. Furthermore, with the increasing complexity of collaborative medical research projects, maintaining a strong framework for medical research oversight is essential for safeguarding patient rights in studies. Ultimately, research ethics and patient safety must remain at the forefront as we navigate the evolving landscape of medical research.
Ensuring the well-being of individuals involved in clinical studies is paramount, especially as the dynamics of biomedical inquiry evolve. The imperative for participant protection has led to a meticulous review process overseen by institutional review boards (IRBs), which guarantee that ethical considerations are embedded in every research endeavor. As we witness collaborations among various research facilities, the harmonization of protocols is crucial to uphold patient safety standards. Meanwhile, ongoing funding challenges threaten to disrupt these safeguards, underscoring the urgent need for robust medical research governance. Ultimately, ethical oversight remains central to preserving the integrity and trust essential for the advancement of healthcare innovation.
The Impact of Funding Cuts on Medical Research Oversight
Funding cuts in medical research significantly impact the oversight mechanisms designed to protect patient safety. These reductions in financial resources often result in the cancellation or postponement of essential research projects. When studies are stalled, patient safety protocols can be inadequately enforced, leading to potential ethical breaches in research practices. Institutions rely heavily on federal grants, such as those from NIH, to maintain their oversight functions and ensure compliance with IRB regulations. With reduced funding, many research teams face challenges in meeting the rigorous standards set for patient protection.
Moreover, the halt in federal research funding disrupts ongoing collaborations among multiple sites, which are crucial for advancing medical innovation. For example, if an IRB overseeing a shared study cannot meet its operational needs, it may not be able to fulfill its responsibilities to monitor patient safety consistently. This can create gaps in the research process where participant welfare is compromised, echoing historical lessons learned through past unethical practices. The ripple effects of funding cuts reach beyond immediate financial impacts; they can erode public trust in medical research, discouraging participation in critical studies.
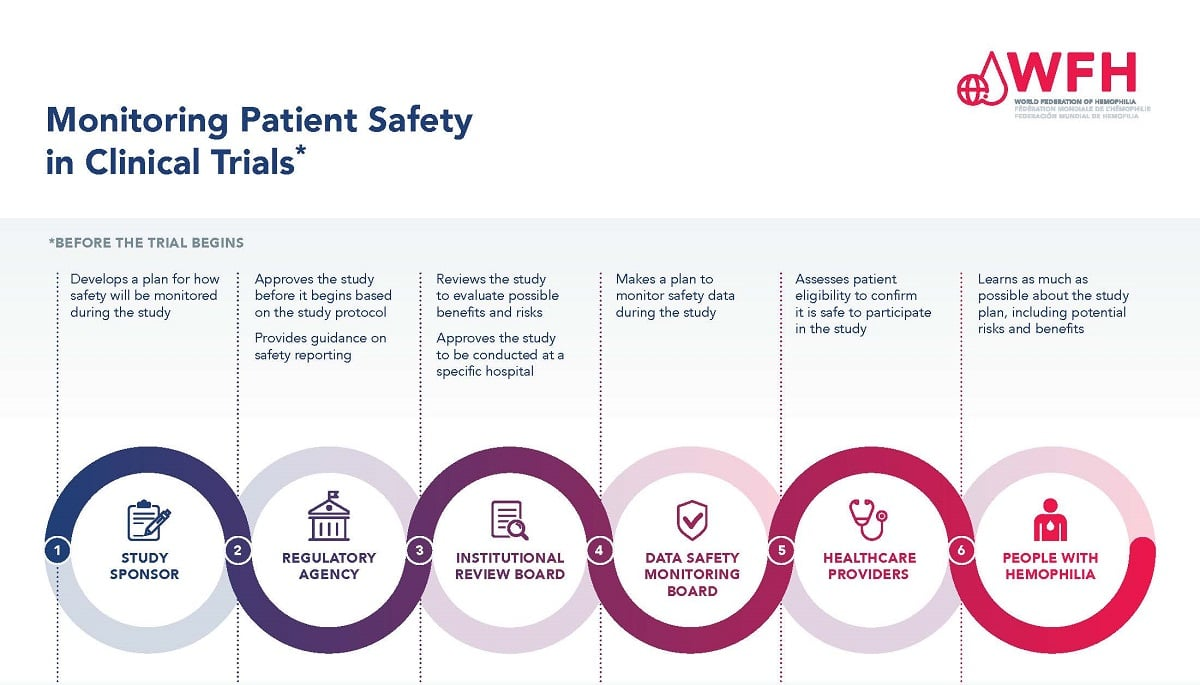
Understanding the Role of Institutional Review Boards (IRBs)
Institutional Review Boards (IRBs) are vital in protecting the rights and welfare of individuals enrolled in clinical trials. They serve as ethical guardians, ensuring that all research complies with applicable laws and ethical standards. The review process involves a detailed assessment of study designs, potential risks to participants, and the informed consent process. Each proposal undergoes scrutiny to identify whether adequate protections are in place for participants, particularly vulnerable populations. By implementing rigorous oversight, IRBs play an essential role in enhancing patient safety in medical research.
Beyond just evaluating proposals, IRBs also provide ongoing oversight throughout the research lifecycle. This includes monitoring adverse events related to the research and ensuring that the study adheres to the approved protocols. Their work is critical for maintaining the integrity of the research process, fostering trust between researchers and participants. Without the robust input from IRBs, many studies may overlook essential patient safety considerations, potentially leading to harmful outcomes. The importance of these boards cannot be understated, especially in a landscape influenced by funding cuts which threaten their operational effectiveness.
Patient Rights in Medical Studies
Understanding patient rights in medical research is paramount to fostering ethical research practices. Each individual involved in a clinical trial must be fully informed about their participation, which includes knowledge of the study’s aims, potential risks, and benefits. This informed consent process ensures that participants are making knowledgeable decisions about their involvement, emphasizing their autonomy and rights. Patients must feel empowered to ask questions and express concerns, and this is where the role of investigators and IRBs is critical in facilitating open dialogue.
However, funding cuts pose challenges to the effective communication of patient rights and can limit the resources available for training researchers on ethical practices. When institutions struggle financially, comprehensive patient education programs may be underfunded, leading to a lack of transparency in the research process. Strengthening patient awareness and rights is essential, not just for compliance but also for fostering an environment of trust between researchers and participants. This trust is essential for the success of medical studies and for ensuring that participants feel safe and secure throughout the research process.
Research Ethics: Balancing Innovation and Patient Safety
The field of medical research is driven by the pursuit of innovation; however, this must be balanced with the imperative to ensure patient safety. Ethical frameworks guide researchers in navigating this delicate balance. Researchers must prioritize the well-being of participants, questioning not only the scientific validity of their studies but also the ethical implications and potential risks involved. Ethical guidelines serve as a blueprint for maintaining integrity while pushing the boundaries of knowledge.
Unfortunately, when funding cuts occur, the emphasis on ethics can be overshadowed by financial constraints. Institutions may rush to conduct studies without adequate oversight or sufficient ethical reviews due to a lack of resources. This can lead to insufficient safeguarding of participant welfare, which compromises the ethical foundation of the research. It is vital for researchers, ethics boards, and funding bodies to collaborate to ensure that innovation does not come at the expense of the rights and safety of those who enrich studies through their participation.
The Importance of Collaborative Research in Patient Safety
Collaborative research efforts are essential for the advancement of medical knowledge, especially in large-scale studies involving multiple sites. When hospitals and universities work together, they share valuable insights and resources, which ultimately contributes to enhanced patient safety. The establishment of networks like SMART IRB demonstrates how collaborative platforms enable institutions to streamline research oversight and focus on protecting participants’ rights. These collaborations are crucial in ensuring that diverse patient populations are represented and that findings are applicable across different demographics.
However, such collaborations are jeopardized by funding cuts, which can hinder partnerships and limit the capacity for multi-site studies. When financial resources are scarce, institutions may be unable to engage fully in collaborative efforts, diminishing the collective ability to prioritize patient safety. The integrity of research is built on cooperation and shared accountability, and safeguarding funding for these collaborative initiatives is imperative for maintaining robust protections for research participants.
Historical Lessons in Medical Research Ethics
History provides valuable lessons in medical research ethics, especially concerning patient safety. Past atrocities, such as the Tuskegee syphilis study and the Nazi experiments during World War II, highlight the need for stringent oversight and ethical consideration in research practices. These events sparked significant changes in how studies are conducted, leading to the establishment of regulatory frameworks meant to protect patients’ rights and welfare. Understanding this context can enable today’s researchers and IRBs to avoid repeating past mistakes by prioritizing patient safety.
Additionally, engaging with historical cases underscores the vital role of informed consent and transparent communication in ethical research. It serves as a stark reminder that patient trust is earned and must be diligently protected through ethical practices. The ethical framework that emerged in response to past wrongs continues to shape modern research regulations, ensuring that patient safety remains at the forefront of the medical research agenda.
Patient Safety in Regulatory Practices
Regulatory practices are pivotal in safeguarding patient safety throughout the research process. Oversight bodies, like the FDA and NIH, establish and enforce regulations that ensure research involving human subjects adheres to ethical standards, minimizing risk and enhancing participant protections. This regulatory landscape is critical as it sets the foundation for researchers to conduct trials responsibly while maintaining accountability to the public and the scientific community.
Furthermore, regulatory practices must continuously evolve to address new challenges and technologies in medical research. As the landscape changes, so do the ethical considerations surrounding patient safety. Ensuring that researchers are well-informed about these regulations is crucial; funding cuts can hinder training programs that are essential for compliance. Protecting patient safety requires a commitment to upholding these regulatory standards, even amid financial constraints, and it is imperative that institutions actively prioritize this integrity.
The Future of Patient Safety in Medical Research
Looking forward, the commitment to ensuring patient safety in medical research will demand a renewed focus on ethical practices and adequate funding. As society grapples with the complexities of emerging medical technologies and research methodologies, the protection of participants must remain paramount. This involves not just adequate regulatory frameworks but also cultural shifts within research institutions to place patient welfare at the core of their missions.
Additionally, advocacy for sustained funding in medical research is essential for maintaining robust oversight and ethical standards. Engaging with policymakers and stakeholders about the importance of patient safety in research can help prevent future funding cuts from undermining these critical protections. A collective responsibility exists among researchers, institutions, and the community to ensure that medical research continues to advance while prioritizing the utmost care and consideration for those who contribute to its success.
Frequently Asked Questions
What is the role of institutional review boards (IRB) in patient safety in medical research?
Institutional Review Boards (IRBs) play a critical role in patient safety in medical research by reviewing and approving research proposals to ensure the ethical treatment of participants. They assess the benefits and risks of research, ensure informed consent, and monitor ongoing studies to protect patient rights and safety throughout the research process.
How do funding cuts affect patient protection in medical research?
Funding cuts can severely impact patient protection in medical research by limiting the resources available for IRBs to effectively oversee studies. Reduced funding may lead to disrupted oversight, increased risks to participant safety, and potential for unethical research practices, ultimately compromising the integrity and trust in medical studies.
What are the implications of research ethics on patient safety in studies?
Research ethics are essential for safeguarding patient safety in studies, as they establish guidelines for conducting research responsibly. Adhering to ethical principles ensures that participants are treated with respect, informed about the research, and protected from harm, thereby enhancing the overall quality and trustworthiness of medical research.
What are patient rights in studies and how do they relate to patient safety in medical research?
Patient rights in studies include the right to informed consent, the right to withdraw from a study at any time, and the right to know about any potential risks involved. These rights are fundamental to safeguarding patient safety in medical research, ensuring that participants are treated ethically and their welfare is prioritized.
How does medical research oversight impact patient safety?
Medical research oversight, primarily conducted by IRBs, plays a vital role in enhancing patient safety by ensuring compliance with ethical standards and regulations. Oversight mechanisms evaluate research protocols, monitor studies for adverse events, and safeguard participant welfare, thereby reinforcing public trust in the research process.
| Key Point | Explanation |
|---|---|
| Funding Cuts Affect Patient Safety | Over $2 billion in federal research grants were cut by the Trump administration, disrupting patient safety efforts in medical research. |
| Importance of IRBs | Institutional Review Boards (IRBs) ensure compliance with ethical standards and the safekeeping of patients in research studies. |
| Consequences of Halts | Halting studies midstream can damage public trust and cause potential harm to research participants, affecting their well-being. |
| Historical Context | Past medical ethics violations highlight the necessity for IRBs to protect patient rights and safety. |
| Collaboration in Research | SMART IRB facilitates collaboration among hospitals and universities, improving the efficiency of innovative research. |
| Future of Patient Safety | Ongoing financial support is vital to maintaining the safety and rights of patients in medical research. |
Summary
Patient safety in medical research is paramount for the ethical conduct of clinical trials and research studies. The recent funding cuts have severely impacted the capabilities to safeguard patients participating in these studies. With the current halt in federal funding, many research efforts have been disrupted, leading to increased risks for patients and a potential decline in public trust in the research enterprise. As such, a robust commitment to funding and oversight is essential to ensure that patient safety remains a top priority in the evolving landscape of medical research.