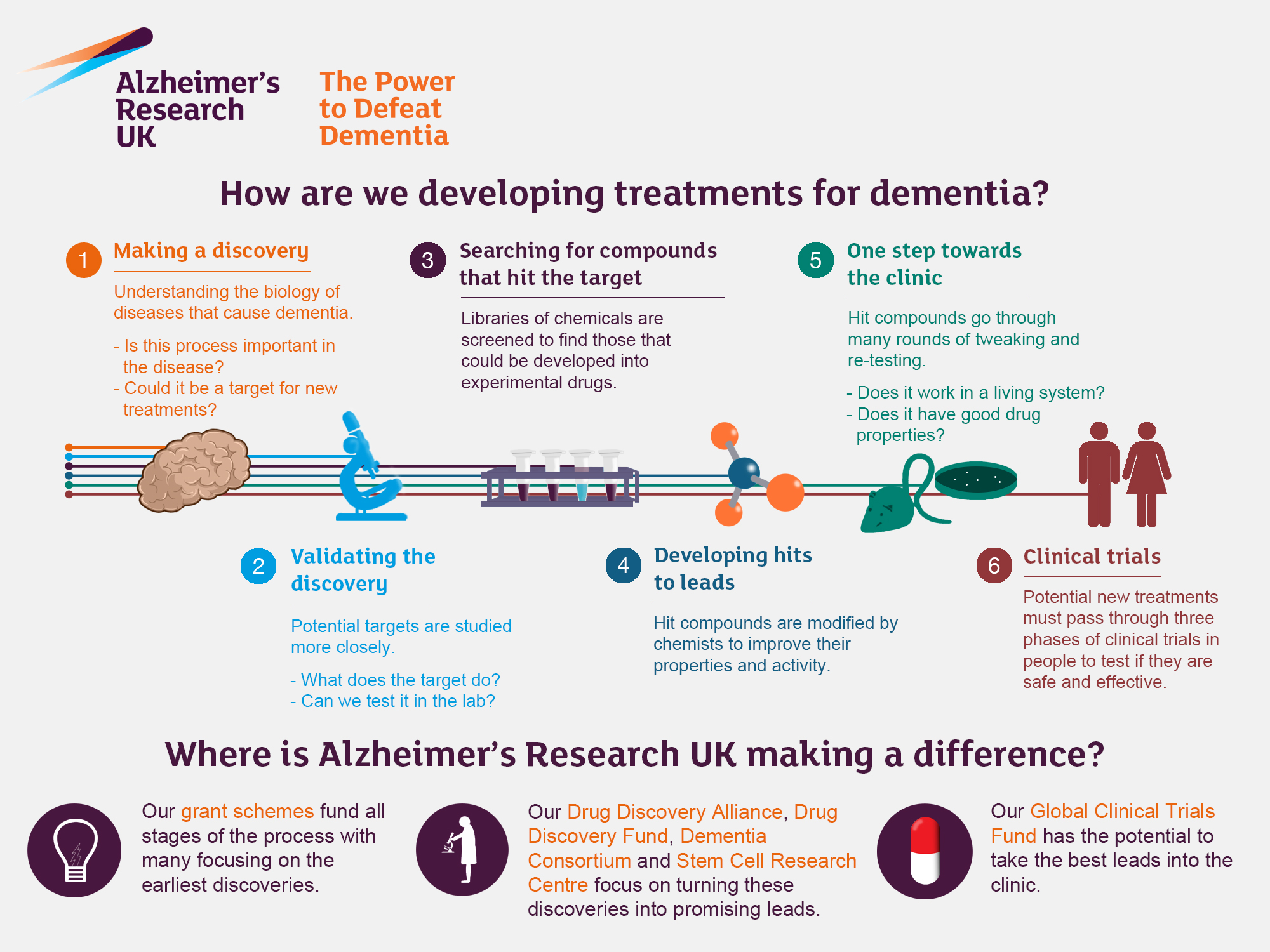
Alzheimer’s research is at the forefront of neurological innovation, shedding light on the complex interactions within the brain’s immune system, primarily through the study of microglia. Renowned neuroscientist Beth Stevens is transforming our understanding of these vital cells, which play a critical role in maintaining brain health by removing dead cells and modulating synapse connections. Her groundbreaking work reveals that dysfunction in microglial activity can contribute to the progression of Alzheimer’s disease and other neurodegenerative diseases. With the alarming increase in Alzheimer’s cases projected to double by 2050, Stevens’ research could pave the way for revolutionary treatments that not only target the disease but also harness the brain’s innate immune responses. As we continue to explore this field, the insights gained from such studies will be crucial in improving the lives of millions affected by Alzheimer’s treatment challenges today.
Research into Alzheimer’s disease, a prevalent form of dementia, is revolutionizing our approach to understanding neurodegenerative conditions. By investigating the role of microglial cells—key players in the brain’s immune response—scientists like Beth Stevens are uncovering the underlying mechanisms that contribute to cognitive decline. This exciting field of exploration is not just about addressing Alzheimer’s but also looks into how immune pathways impact overall brain health. The potential to develop effective therapies rests on integrating findings from basic research with clinical applications, highlighting the importance of curiosity-driven science. Understanding the balance of synapse pruning by microglia opens new avenues for early detection and intervention in debilitating diseases that affect millions globally.
The Role of Microglia in Neurodegenerative Diseases
Microglia, the resident immune cells of the brain, play a crucial role in maintaining neuronal health and homeostasis. They continuously monitor the brain’s microenvironment, responding to any signs of injury or disease. In the context of neurodegenerative diseases, such as Alzheimer’s and Huntington’s, the behavior of microglia can become dysregulated. Recent research indicates that aberrant microglial pruning can lead to excessive removal of synapses, which disrupts the normal communication pathways among neurons, further exacerbating cognitive decline in diseases like Alzheimer’s. Understanding the mechanics of microglial activity is essential for developing therapies that can effectively target these processes and restore healthy brain function.
Moreover, the insights gained from studying microglia have profound implications for uncovering biomarkers that could aid in the early detection of neurodegenerative diseases. By identifying the molecular changes that occur within microglia as a response to pathological conditions, researchers are laying the groundwork for diagnosing Alzheimer’s disease at much earlier stages. This proactive approach not only holds promise for individual patients but also aims to alleviate the broader impact of these diseases on public health.
Beth Stevens’ pioneering research on microglia has shifted the paradigm in understanding their dual role in the brain. While they are vital for clearing dead cells and pathogens, when the balance is disrupted, they can contribute to the pathology of neurodegenerative diseases like Alzheimer’s. Stevens emphasizes that microglial dysfunction is not merely a byproduct of disease but rather a significant player in neurodegeneration. By highlighting their importance, she has opened new avenues for research targeting these immune cells, suggesting that modulating microglial activity might lead to innovative treatment protocols for Alzheimer’s, ultimately improving outcomes for millions suffering from this condition.
Advancements in Alzheimer’s Research Driven by Curiosity
Curiosity-driven research has been at the heart of many groundbreaking discoveries in medicine, particularly in Alzheimer’s research. Beth Stevens exemplifies how following scientific inquiry without the immediate expectation of results can lead to a deeper understanding of complex diseases. Her work with microglia underscores the importance of funding curiosity-led initiatives that explore fundamental questions about brain health. Instead of focusing solely on direct applications, Stevens’ exploration of how the brain’s immune cells interact during development has spurred transformative insights into the pathogenic processes involved in Alzheimer’s disease.
As Stevens notes, much of this foundational research is supported by grants from the National Institutes of Health (NIH), which recognize the value of fostering a scientific environment where exploration can thrive. It is this investment in basic science that has allowed researchers to unlock the intricate mechanisms of microglial function and its implications for Alzheimer’s treatment, laying a solid framework for future therapeutic interventions.
As the global population ages, the urgency of understanding and combating Alzheimer’s disease intensifies. Stevens’ research illustrates that breakthroughs often arise from a combination of curiosity and rigorous scientific methodology. By prioritizing the study of microglia within the broader context of neurodegenerative disease frameworks, Stevens and her colleagues have begun to unravel the complexities of synaptic dysregulation and its relation to cognitive decline. These initiatives not only drive forward therapeutic strategies but also inspire a new generation of scientists to explore the unexplored realms of brain immunity and neurodegeneration.
Innovative Treatments Emerging from Microglial Research
The exploration of microglial cells is leading to promising innovations in treatments for Alzheimer’s disease. With the recognition of microglia as essential players in the brain’s immune response, researchers are now focusing on how to manipulate their activity for therapeutic benefit. The work of Beth Stevens has been pivotal in demonstrating that abnormalities in microglial pruning can contribute significantly to the progression of Alzheimer’s. Because of this, pharmaceutical companies are exploring compounds that can regulate microglial functions, aiming to restore their natural protective roles.
These new strategies may include the use of drugs that enhance microglial activity, allowing them to better clear harmful proteins associated with Alzheimer’s, such as amyloid beta. Early experimental treatments targeting microglia have shown encouraging results in laboratory settings, suggesting that they could precede the development of more traditional Alzheimer’s treatments that focus solely on symptom management. As researchers refine these approaches, there’s a growing optimism that microglial-targeted therapies could substantially alter the course of Alzheimer’s disease and improve the quality of life for those affected.
Additionally, the identification of specific biomarkers linked to microglial activity holds potential for enhancing Alzheimer’s diagnostics. By utilizing advanced imaging techniques coupled with genetic profiling, scientists can gain insights into the status of microglial cells in patients, leading to earlier diagnosis and tailored therapeutic interventions. As research progresses, the development of a biomarker-driven framework could help in stratifying patients based on their microglial profiles, thus allowing for a more personalized approach to Alzheimer’s treatment that precisely targets the underlying pathogenic mechanisms.
Understanding the Brain’s Immune System Through Microglia
Microglia are often referred to as the brain’s own immune system, serving not only to defend against pathogens but also to maintain the overall health of neuronal networks. The evolution of microglial research has revealed fascinating insights into their role beyond mere guardianship. They actively participate in synaptic pruning during development, a crucial process for healthy brain circuit formation. These functions underscore the importance of microglia not only in responding to injury or disease but in sculpting the brain’s architecture over its lifespan.
As researchers delve deeper into the complex signaling pathways that govern microglial function, they are beginning to understand how various factors, including aging and neurodegenerative processes, can disrupt their activity. This understanding could lead to significant advancements in targeted Alzheimer’s therapies, aiming not only to address symptoms but to restore normal immune function and homeostasis in the brain.”},{
Frequently Asked Questions
How do microglia contribute to Alzheimer’s research?
Microglia, the brain’s immune cells, play a crucial role in Alzheimer’s research by managing inflammation and clearing damaged cells. Recent studies, led by researchers like Beth Stevens, have shown that aberrant microglial activity can contribute to the progression of Alzheimer’s disease, which paves the way for potential new treatments and biomarkers.
What role do neurodegenerative diseases play in Alzheimer’s research advancements?
Neurodegenerative diseases, including Alzheimer’s, are at the forefront of biomedical research. Insights gained from studying these diseases can reveal the underlying mechanisms of brain function, promoting the development of targeted therapies and enhancing our understanding of conditions like Alzheimer’s and Huntington’s disease.
Who is Beth Stevens and why is her work significant in Alzheimer’s research?
Beth Stevens is a prominent neuroscientist known for her groundbreaking research on microglial cells in the brain’s immune system. Her findings have transformed how scientists understand the connections between microglial activity and the development of Alzheimer’s disease, leading to innovative approaches in treatment strategies.
What is the relationship between the brain’s immune system and Alzheimer’s treatment?
The brain’s immune system, primarily composed of microglia, is integral to Alzheimer’s treatment strategies. Research suggests that enhancing microglial function or mitigating their aberrant activity could effectively combat neurodegenerative diseases, offering new avenues for developing effective therapies against Alzheimer’s.
How can microglial dysfunction lead to Alzheimer’s disease?
Microglial dysfunction can lead to Alzheimer’s disease through improper synaptic pruning and persistence of inflammatory processes. This imbalance contributes to neuronal damage and cognitive decline, underscoring the importance of targeting microglial mechanisms in Alzheimer’s research for potential therapeutic interventions.
What impact does foundational research have on Alzheimer’s disease therapies?
Foundational research, like that conducted by Beth Stevens and others, provides essential insights into the biological mechanisms of Alzheimer’s disease. Such research lays the groundwork for developing effective therapies and identifying early biomarkers, ultimately advancing the fight against Alzheimer’s.
Why is it important to investigate microglial activity in neurodegenerative diseases?
Investigating microglial activity is vital in neurodegenerative diseases because these immune cells significantly influence brain health. Understanding their role can reveal pathophysiological processes underlying diseases like Alzheimer’s, informing the development of targeted treatments to improve patient outcomes.
What are the future implications of Alzheimer’s research on microglia?
The future implications of Alzheimer’s research on microglia are vast, with potential advances in early diagnosis and treatment strategies. By further understanding microglial roles, researchers can develop novel therapies aimed at correcting dysfunctional immune responses in the brain, leading to significantly improved management of Alzheimer’s disease.
How might new biomarkers developed from Alzheimer’s research improve diagnostics?
New biomarkers derived from Alzheimer’s research could facilitate earlier detection of the disease, enabling timely interventions. By identifying specific molecular signatures associated with microglial dysfunction, clinicians can better diagnose Alzheimer’s and monitor disease progression.
| Key Points | |
|---|---|
| Neuroscientist Beth Stevens’ research on microglial cells | Transforming understanding of brain immune system and synapse pruning |
| Aberrant pruning linked to Alzheimer’s and other disorders | Potential for new medicines and biomarkers |
| 7 million Americans estimated to live with Alzheimer’s | Annual cases expected to double by 2050, tripling care costs |
| Research driven by federal funding and curiosity-driven science | Foundational research leads to critical discoveries and treatment avenues |
| MacArthur ‘genius’ award recipient in 2015 | Relevance of basic science in understanding disease mechanisms |
Summary
Alzheimer’s research is crucial as it delves deep into the mechanisms behind this devastating disease. Through the groundbreaking work of scientists like Beth Stevens, we gain insights into the role of microglial cells in brain health and the potential ramifications of their malfunction in diseases like Alzheimer’s. This evolving understanding has the power to reshape treatment options for millions and highlights the importance of ongoing scientific inquiry and funding in tackling one of society’s most pressing health challenges.